Ideal Gas Law Calculator
Blog post description.
Wiratama
11/15/20251 min read
Ideal Gas Law – Definition
The ideal gas law describes the relationship between pressure, volume, temperature, and the amount of gas. It provides a useful approximation for gases at low pressure and moderate temperature, where gas molecules behave nearly independently and intermolecular forces are negligible.
Background Theory
The ideal gas law is expressed as:
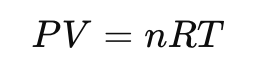
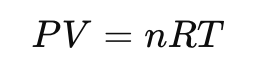
Where:
P = pressure (Pa)
V = volume (m³)
n = number of moles (mol)
T = absolute temperature (K)
R = universal gas constant ≈ 8.314 J/(mol·K)
The equation is derived from combining Boyle’s law, Charles’ law, and Avogadro’s law. It is widely used across physics, chemical engineering, thermodynamics, and HVAC calculations. While real gases deviate from ideal behavior at high pressures or low temperatures, the ideal gas law remains accurate for many engineering applications.
How This Calculator Works
This calculator solves the ideal gas law for any missing variable—pressure, volume, moles, or temperature—by:
Letting you choose which variable to calculate
Taking input values for the remaining three variables
Applying the rearranged ideal gas law formula
Displaying the computed value in scientific notation for accuracy
This tool provides a quick and reliable way to analyze gas behavior in thermodynamic systems, process equipment, and laboratory conditions.
